
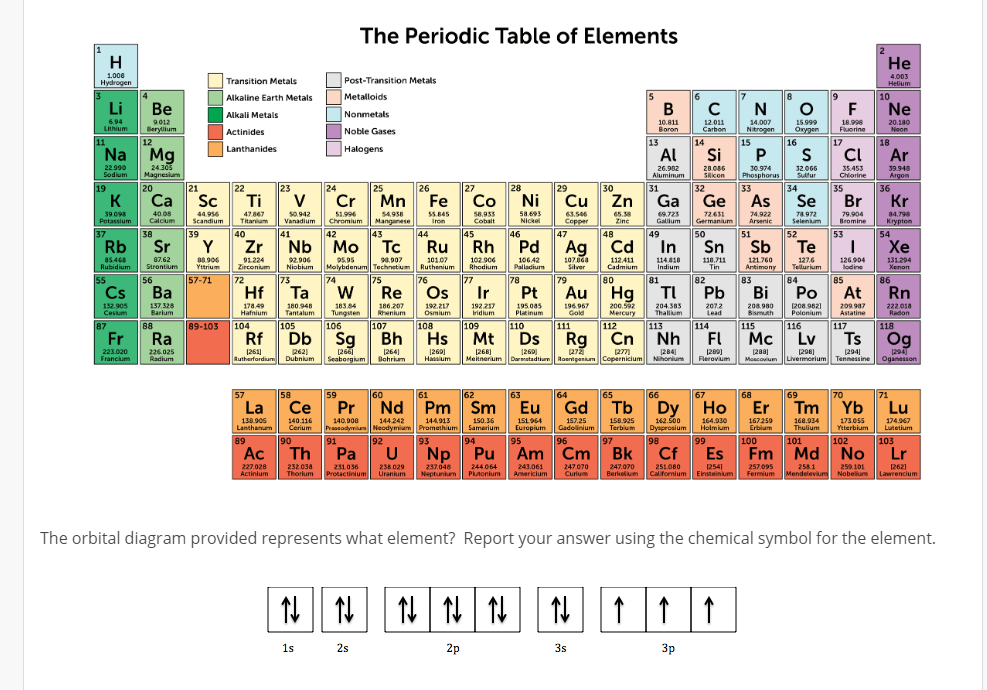
The chemical symbol Na derives from the alternative name, natrium or natronium, proposed by Ludwig Wilhelm Gilbert, which is used in other languages. Davy proposed the name sodium, after the word soda, which is believed to derive from the Arabic word suda meaning headache, to which soda (i.e. It was first isolated in 1807 through the electrolysis of NaOH by Sir Humphry Davy. However, as it is highly reactive, sodium metal is not found naturally. Sodium compounds such as salt (NaCl), soda (Na 2CO 3) and lye (NaOH) have been known for millennia, and used domestically in food, medicines, soap making and various other applications.
Lithium metal in vial, lithium-ion batteries and Lithicarb medication (Li 2CO 3) Lithium salts are also used as psychiatric medication.
There are many uses for lithium, including in glass and ceramics, lubricants, batteries, as a rocket propellant, and in the production of nuclear energy and weapons. The metal in these compounds was subsequently named lithium, and was first isolated by William Thomas Brande in 1821. The alkaline compounds were named lithina by Jons Jakob Berzelius, in whose lab Arfwedson worked, from the Greek lithos meaning stone, reflecting their discovery from a solid mineral. In 1817, Johan August Arfwedson identified that it contained a new element as it produced similar alkaline compounds to sodium and potassium, but which were less soluble. The lithium-containing mineral petalite was discovered in 1800 by Jose Bonifacio de Andrada e Silva. Whilst hydrogen is commonly found in water and hydrocarbons, its main use as an element is in processing fossil fuels and fats and oils, and in the production of ammonia for food production. Two years later Antoine Lavoisier named the element hydrogene after the Greek hydro- meaning water, and -genes meaning to create or produce. In 1781, he found that water was produced when hydrogen was burned in oxygen. Hydrogen is released in reactions between acids and metals, and was identified as a distinct substance in these reactions by Henry Cavendish in 1766. What elements are in Group 1 – Hydrogen and Alkali Metals?Ĭlick on the following elements to learn more about them:


 0 kommentar(er)
0 kommentar(er)
